Stem cell charity Anthony Nolan together with Blood Cancer UK and Lymphoma Action are calling on NICE to reconsider its decision to remove the CAR T-cell therapy brexucabtagene autoleucel (brexu-cel) as a treatment for mantle cell lymphoma after five years of it being available on the NHS in England and Wales.
Around 600 people are diagnosed with mantle cell lymphoma each year in the UK. It is a type of blood cancer that affects white blood cells called B lymphocytes, whose main job is to help the body fight infections.
Brexu-cel, also known by the brand name Tecartus, is a type of treatment called CAR T-cell therapy that uses cells from a patient’s own immune system to fight cancer. It is the only CAR T-cell therapy available for relapsed or refractory mantle cell lymphoma and can be a potentially lifesaving option for people who haven’t responded to other treatment.
The removal of Tecartus leaves patients in this position with very few other possible treatments, most of whom will only have the option of receiving palliative care.
Emily John, CAR T- cell therapy specialist nurse at Anthony Nolan, said: “We are deeply disappointed by NICE’s decision. As a specialist nurse, I’ve seen first-hand how Tecartus has transformed the lives of patients living with mantle cell lymphoma, offering them a lifeline when other treatment options haven’t worked.”
“The removal of the only CAR T-cell therapy available for people with mantle cell lymphoma is incredibly troubling and a backward step for NHS care. We urge NICE and the manufacturer to find a solution so people with relapsed mantle cell lymphoma don’t lose access to this potentially lifesaving treatment.”
Dr Rubina Ahmed, director of research, policy and services at Blood Cancer UK, said:
“For some people with mantle cell lymphoma, whose cancer has come back or hasn’t responded to previous treatment, this CAR T-cell therapy offers a last hope of a cure. We recognise that NICE decisions involve complex clinical and economic considerations, but we are concerned about what this could mean for patients who have very limited alternatives.
“That’s why we have formally submitted an appeal, raising questions about how this treatment has been assessed and the implications of this decision for patients. It’s vital that advances in blood cancer treatment are reflected in the options available to people in practice.”
Ropinder Gill, chief executive at Lymphoma Action said:
“The primary focus of Lymphoma Action remains the people living with mantle cell lymphoma who may benefit from this treatment. We have received multiple enquiries from really concerned and anxious members of our community and spoken to people who saw this CAR T-cell therapy as a potential treatment for their mantle cell lymphoma. There is now a lot of distress that this treatment could be removed as an option.
“It is vital that decision makers understand the impact that this decision could have on people affected by relapsed or refractory mantle cell lymphoma, which is why we have joined colleagues in submitting a formal appeal against the NICE decision.”
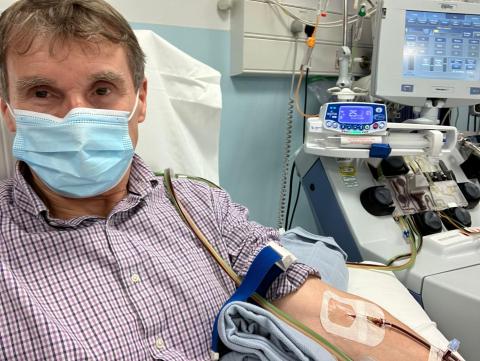
Paul Madley, 66 from Cardiff, who was diagnosed with stage 4 mantle cell lymphoma in 2021, has also expressed his extreme disappointment. He said: “After receiving my diagnosis, I had multiple rounds of chemotherapy and went into remission the following year. I also had a stem cell transplant to prolong my remission, but unfortunately my cancer returned in July 2024.”
“Fortunately, I was put forward to receive CAR T-cell therapy and began treatment in Autumn 2024. By March 2025, I was given the wonderful news that I was in remission and that’s where I currently remain. I lead a full and active lifestyle - I have returned to work as a consultant Chartered Surveyor 3-4 days a week, walk my dog most mornings, play golf and generally enjoy my life as I did before my illness.”
“To therefore be told that NICE have decided to remove this treatment I find truly unbelievable. I have a whole host of different emotions on their decision – sadness, incredible disappointment and anger to name but a few. This treatment is helping to prolong lives of people like me – without it goodness knows where I would be.”
Anthony Nolan, Blood Cancer UK and Lymphoma Action have jointly submitted an appeal against NICE’s final draft guidance. NICE have confirmed its recommendation should not affect any mantle cell lymphoma patients who started Tecartus treatment before the final draft guidance was published on 24th December 2025. We encourage patients who have concerns about their treatment to speak to their medical team.